Term Used to Describe Noble Gases
They will be replaced shortly. Helium Neon Argon Krypton Xenon and Radon are noble gases.

Group 18 Elements Noble Gases Physical And Chemical Properties Noble Gas Free Tuition
The elements in group 18 are the noble gases helium neon argon krypton xenon and radon.

. The noble gases were previously known as inert gases because of their perceived lack of participation in any chemical reactions. Neil Bartlett at the University of British Columbia proved this assumption to be false. C gases possess kinetic energy.
Neil Bartlett at the University of British Columbia. They include Helium neon argon xenon among others. A gas molecules lose net kinetic energy as they collide.
It can be made by reacting sodium with chlorine gas. Nobles gases are group 8 elements they have an octet configuration with a maximum number of electrons in the outer most energy level. A gas molecules lose net kinetic energy as they collide.
The reaction takes place at room temperature and pressure. Your source for the latest research. The term noble refers to.
They are generally very stable colorless and odorless. The only elements that exist in nature as uncombined atoms are the noble gases. The oxidation state is the charge associated with an atom.
The group consists of 7 elements. The noble gases are helium argon krypton xenon and radon in order of their mass. All the chemical elements in this group are monoatomic gases colorless and odorless.
Noble gases are highly unreactive due to their full outer shell of electrons. The term non-metals is used to classify the elements H C N P O S and Se. What term describes boron.
To predict the three-dimensional shaped of molecules What shape would you expect a simple carbon-containing compound to have if the carbon atom has sp2 hybridization. The Noble gases are called that because they are naturally resistant to forming compounds with other elements. Helium neon argon krypton xenon and radon.
The noble gases were previously referred to as inert gases but this term is not strictly accurate because several of them do take part in chemical reactions. Noble gases are a group of nonmetals in group 18 that are often described as chemically inert - they are colorless odorless and highly unreactive. These elements are described as mono-atomic molecules.
Theyre also known as inert gases for this reason. Only a few of them combine with other elements and then only with fluorine the most reactive of all substances. They earned the name noble because they were assumed to be nonreactive since they have filled valence shells.
They earned the name noble because they were assumed to be nonreactive since they have filled valence shells. In chemistry the term noble gas refers to any of the six chemically inert gaseous elements in the periodic table which demonstrate similar characteristics. They are nonreactive or have a very low chemical reactivity.
2Nas Cl 2 g 2NaCl s Calculate the volume of chlorine gas in cm3 that reacts to form 234 g of NaCl. Noble gases formally referred to as inert or rare gases make up Group 18 of the periodic table of the elements. These elements are gases at ordinary room temperature and pressure.
Describe the noble gases as being unreactive. All noble gases for instance tend to be highly nonreactive. In the periodic table these elements are located in Group VIII which was initially known as the Zero Group.
This is the result of their arrangement of valence electrons. The word gas was first used by the early 17th. 5 CLE 2020 062042N20 Turn over e Sodium chloride is an ionic salt.
The term noble gas is particularly used for elements of group 18 of the periodic table. This group includes helium neon argon krypton xenon radon and the synthetic element oganesson. The reason for this is that their outmost electron shells valence shells are completely filled so that they have little.
An oxide is a compound composed of a metal and oxygen. The term noble has historically been used in chemistry and prior to that in alchemy to describe the reluctance of certain metals to react chemically and the term noble gas is used to connote that same reluctance. We apologise for the inconvenience but hope that the new images will provide you with an even better learning experience.
Your source for the latest research. Group 0 element s are called noble gases. Monatomic Which is a molecular formula of a diatomic molecule found in Earths atmosphere.
Which of the following best describes the noble gases. The equation for this reaction is shown. They have full outer shells and so are stable leading them to be unreactive.
N2 What do scientists use VSEPR theory for. The crossword clue A noble gas with 5 letters was last seen on the June 27 2021. B there are no forces of attraction or repulsion between gas molecules.
They are called noble gases because they are so majestic that they do not react with anything in general. The elements in group 18 are the noble gases helium neon argon krypton xenon and radon. When grouped together with the monatomic noble gases helium He neon Ne argon Ar krypton Kr xenon Xe and radon Rn these gases are referred to as elemental gases.
Describe the reaction of the noble gases with metals. The noble gases comprise group 18. Choose from a range of topics like Movies Sports Technology Games History Architecture and more.
FAMILIES OF ELEMENTS CONCEPT The term family is used to describe elements that share certain characteristicsnot only in terms of observable behavior but also with regard to atomic structure. Describe the properties preparation and uses of the noble gases. Edexcel Group 0 - the noble gases The group 0 elements the noble gases are all unreactive non-metal gases.
The noble gases make up the last column of elements in the periodic table. They are commonly called Group 18 the inert gases the rare gases the helium family or the neon family. They show trends in their physical properties.
D the kinetic energy is dependent upon temperature. In chemistry the term inert is used to describe a substance that is not chemically reactive. As such in the context of chemistry and materials science these gases are generally referred to as noble gases.

What Are Noble Gases Definition And Properties Noble Gas Gas Ionization Energy
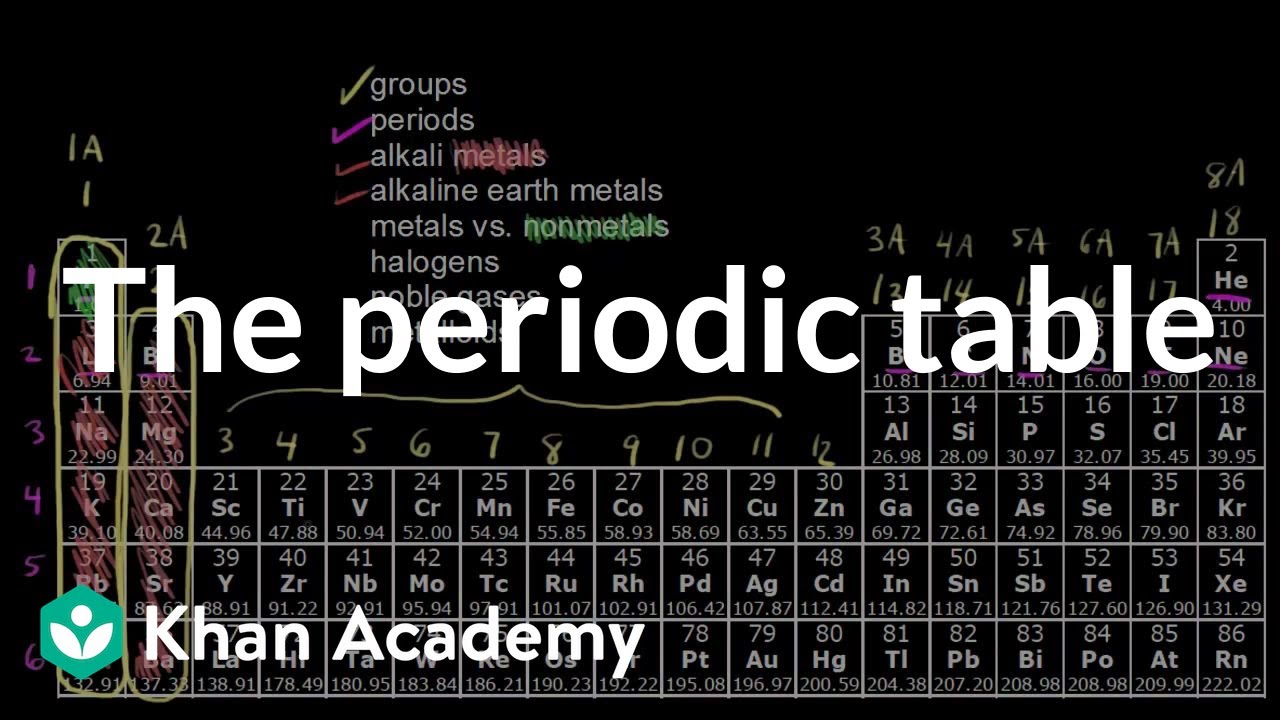
Definitions Of Groups Periods Alkali Metals Alkaline Earth Metals Halogens And Noble Gases How Metals Non Met Periodic Table Chemistry Element Chemistry

Element Infographics The Noble Gases Poster By Compound Interest Noble Gas Infographic Physical Education Bulletin Boards

Comments
Post a Comment